Our Work
Project Description
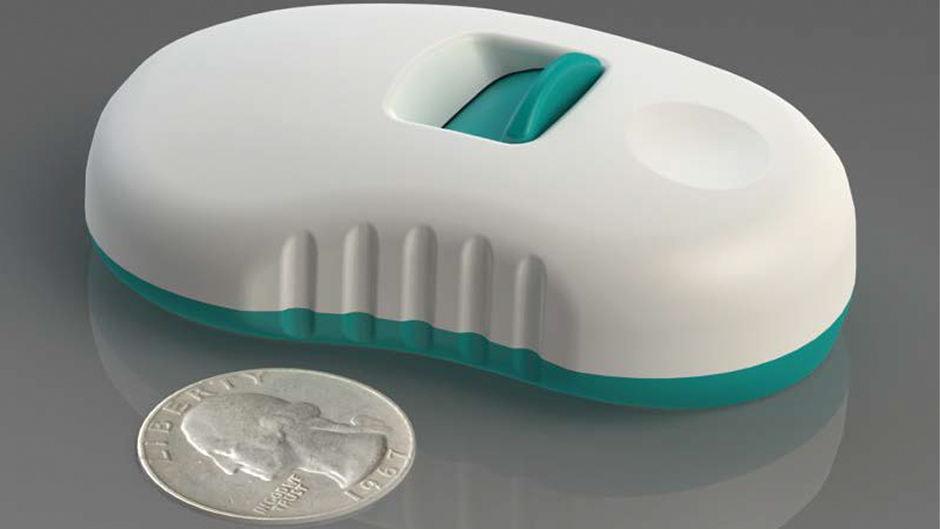
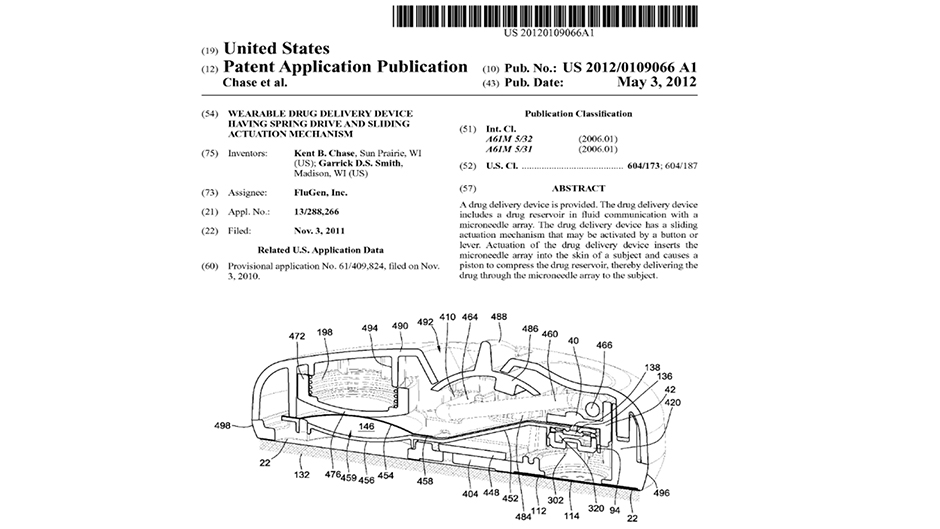
Our client’s team of influenza vaccine experts understood that technology development and design engineering of a novel delivery device capable of painlessly administering influenza vaccine into the skin for increased efficacy was outside of their core competencies. Intense Engineering worked closely with them to not only identify and understand the technical performance and regulatory requirements for the device, but also the business drivers and constraints; this led to a clear understanding of the specific knowledge gaps and risk factors and paved a concise path forward. Through a coordinated technical research, design and validation effort, Intense Engineering completed core technology development to determine the best design to pursue. Our novel designs resulted in several patents. We subsequently developed a complete production design and a series of fully-functioning prototypes suitable for regulated FDA Phase I clinical trials.